Given that a point to sphere force is no longer determined using Coulomb’s Law, a Hydrogen molecule now has four different electrostatic forces at play.
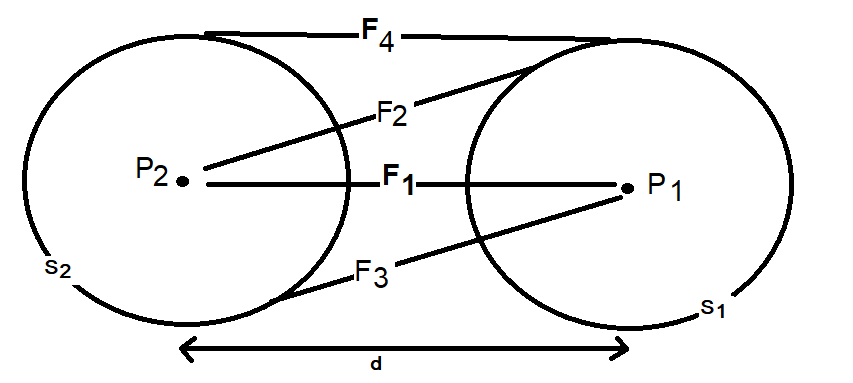
- F1 is the force between the two protons, treated as points. This force is calculated using the Coulombic Law.
- F2 is the force between P2, the Hydrogen proton and C1 the opposing Hydrogen electron cloud treated as a point to sphere interaction. This force is calculated using the “corrected equation”.
- F3 is the force between P1, the Hydrogen proton and C2 the opposing Hydrogen electron cloud treated as a point to sphere interaction. This force is calculated using the “corrected equation”.
- F4 is the force between the two electron clouds, treated as hollow spheres in this example and calculated using the numerical approximation derived earlier.
The point to point and point to sphere force calculations are simple enough to graph over distance but the circle to circle force calculation requires a numerical approximation. As such, a rudimentary programming language was used to calculate all four forces in Figure 8 over varying distance. For simplicity JavaScript was used despite obvious inefficiencies. JavaScript does however allow anyone with a browser to execute the code for the numerical approximation, easily altering parameters for radius, distance and charge.
The JavaScript code to generate graph data is presented in found in Calculator.js referenced by the Calculator page. The resultant force over distance graph is presented below.
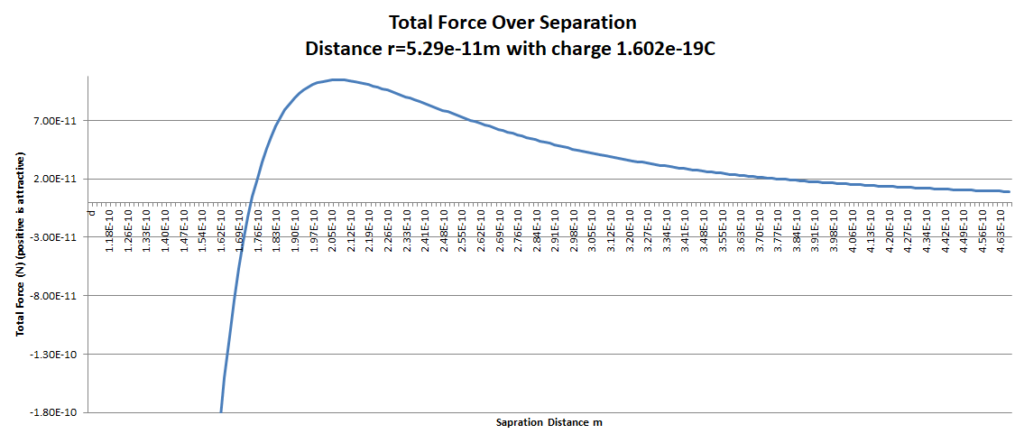
Parameters used in JavaScript calculation to render graph above are below including non scientific notation for pasting into the calculator as required;
- [1]Radius of Hydrogen atom 5.29177 x 10 -11 m (0.000000000053)
- Charge of Hydrogen atom 1.602 x 10-19 C (0.0000000000000000001602)
- Starting Separation distance 0.000000000111300000 m
- Ending Separation distance 0.000000000470300000 m
- Coulombic constant 8.988×109 N⋅m2⋅C−2
- 200 increments
- Points on circle=36
Results;
- Using the corrected equation to model four different electrostatic forces in a Hydrogen atom pair over may distances results in a Lennard Jones curve with a stable distance of 1.7e-10m.
[1] radii data : https://en.wikipedia.org/wiki/Atomic_radii_of_the_elements_(data_page)